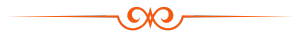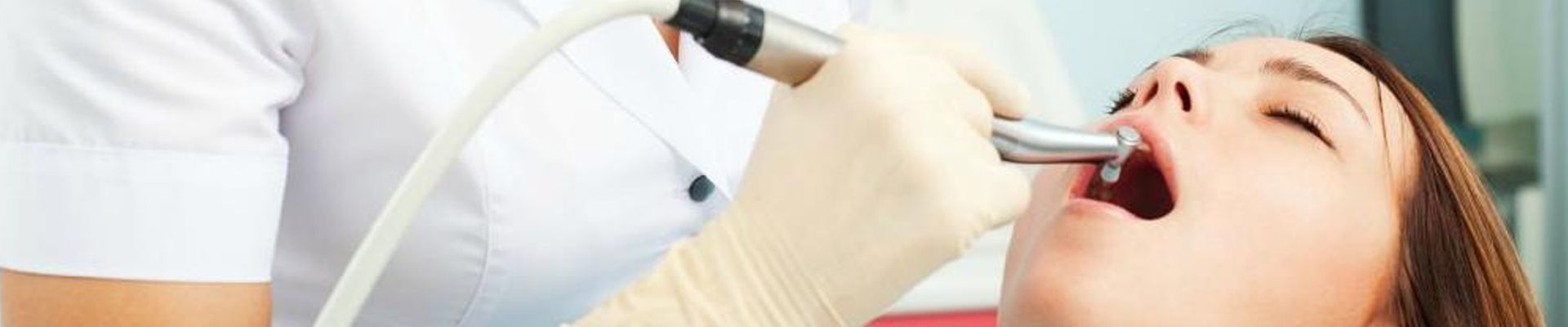
Before the treatment, the clinician should examine the patient: taking a health and dental history (including allergies and sensitivities), observe hard and soft tissues, placement and conditions of restorations, and sometimes x-rays to determine the nature and depth of possible irregularities. If this is not completed prior to the whitening agents being applied to the tooth surface, excessive sensitivity and other complications may occurIn-office bleaching procedures generally use a light-cured protective layer that is carefully painted on the gums and papilla (the tips of the gums between the teeth) to reduce the risk of chemical burns to the soft tissues. The bleaching agent is either carbamide peroxide, which breaks down in the mouth to form hydrogen peroxide, or hydrogen peroxide itself. The bleaching gel typically contains between 10% and 44% carbamide peroxide, which is roughly equivalent to a 3% to 16% hydrogen peroxide concentration. The legal percentage of hydrogen peroxide allowed to be given is 0.1–6%.[where?] Bleaching agents are only allowed to be given by dental practitioners, dental therapists, and dental hygienists.
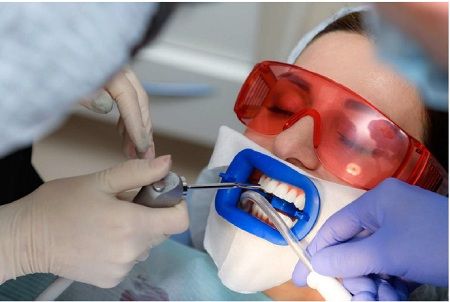
Bleaching is least effective when the original tooth color is grayish and may require custom bleaching trays. Bleaching is most effective with yellow discolored teeth. If heavy staining or tetracycline damage is present on a patient's teeth, and whitening is ineffective (tetracycline staining may require prolonged bleaching, as it takes longer for the bleach to reach the dentine layer), there are other methods of masking the stain. Bonding, which also masks tooth stains, is when a thin coating of composite material is applied to the front of a person's teeth and then cured with a blue light. A veneer can also mask tooth discoloration.
In-chair whitening is faster and more effective in comparison to the take-home bleaching options. Some clinicians also make custom bleaching trays for you, which can take up to a week to create, so that after the whitening treatment is completed, you are able to use these trays in the future for maintenance of your bleaching with at home kits or for the use of desensitising products.
In modern times of today, every individual is making concious efforts to look desirable and presentable. when everyone is in persuit of beauty, no wonder patients comes to dentists desiring perfect smile like billboard models. Having asthetic dentistry evolved largely inpast few years, there are different invasive and non invasive techniques to enhance patient's esthetic dental appearance.
Speaking of Non- invasive in terms of non exposure of inner layers of tooth like dentine, pulp, with relatively not major operative work, many cases can be managed by procedures like Bleaching giving individual whiter and pleasant esthetic appearance retaining his/her natural teeth as they are.
A recent addition to the field is new light-accelerated bleaching agents containing lower concentrations of hydrogen peroxide with a titanium oxide nanoparticle-based catalyst. Reduced concentrations of hydrogen peroxide cause lower incidences of tooth hypersensitivity.[39] The nanoparticles act as photocatalysts, and their size prevents them from diffusing deeply into the tooth. When exposed to light, the catalysts produce a rapid, localized breakdown of hydrogen peroxide into highly reactive radicals. Due to the extremely short lifetimes of the free radicals, they are able to produce bleaching effects similar to much higher concentration bleaching agents within the outer layers of the teeth where the nanoparticle catalysts are located. This provides effective tooth whitening while reducing the required concentration of hydrogen peroxide and other reactive byproducts at the tooth pulp.
Internal bleaching is a process which occurs after a tooth has been endodontically treated. This means that the tooth will have had the nerve of the tooth extirpated or removed through a root canal treatment at the dentist or by a specialist endodontist. Internal bleaching is often sought after in teeth which have been endodontically treated as tooth discolouration becomes a problem due to the lack of nerve supply to that tooth. It is common to have this internal bleaching done on an anterior tooth (a front tooth that you can see when smiling and talking). A way around this is by sealing off the bleaching agent inside the tooth itself and replacing it every few weeks until the desired shade has been achieved. The amount of time between appointments varies from patient to patient and with operator preference until the desired shade has been achieved.[40] Even though this is a great option, the disadvantage of this treatment is a risk of internal root resorption of the tooth that is being internally bleached. This may not occur in every patient or every tooth, and its occurrence is difficult to determine prior to completing the treatment.
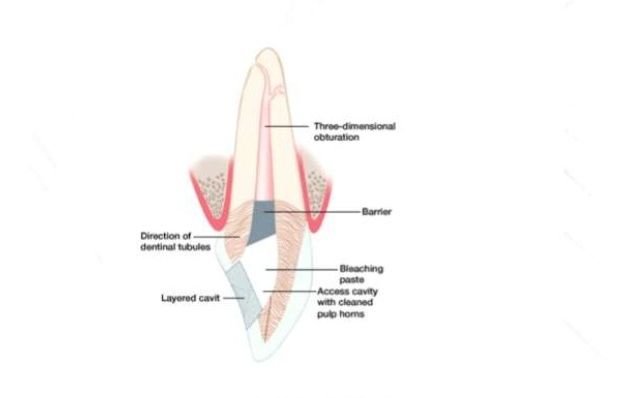
At home tooth whitening products are available from dentists or 'over the counter (OTC). At home whitening methods include; over the counter strips and gels, whitening rinses, whitening toothpastes, and tray-based tooth whiteners. OTC products can be used for milder cases of tooth staining. Home-based bleaching (following manufacturer's instructions) would result in less tooth sensitivity than in-office bleaching.
Strips and gels
The plastic whitening strips contain a thin layer of peroxide gel and are shaped to fit the buccal/labial
surfaces of teeth. Many different types of whitening strips are available on the market, after being
introduced in the late 1980s. Specific whitening strip products have their own set of instructions
however the strips are typically applied twice daily for 30 minutes for 14 days. In several days, tooth
colour can lighten by 1 or 2 shades. The tooth whitening endpoint does depend on the frequency of use
and ingredients of the product.
Whitening gels are applied onto the tooth surface with a small brush. The gels contain peroxide and are
recommended to be applied twice a day for 14 days. The tooth whitening endpoint like that of the
whitening strips.
Rinses
Whitening rinses work by reaction of the oxygen sources such as hydrogen peroxide within the rinse and
the chromogens on or within the tooth. It is recommended to use twice a day, rinsing for one minute. To
see an improvement in shade colour, it can take up to three months.
Toothpastes
Whitening toothpastes are different to regular toothpastes in that they contain higher amounts of
abrasives and detergents to be more effective at removing tougher stains. Some whitening toothpastes
contain low concentrations of carbamide peroxide or hydrogen peroxide which help lighten tooth colour
however they do not contain bleach (sodium hypochlorite). With continuity of use over time, tooth
colour can lighten by one or two shades.
Tray-based
Tray-based tooth whitening is achieved by wearing a fitted tray containing carbamide peroxide
bleaching gel overnight or for two to four hours a day. If manufacturer's instructions are followed, tooth
whitening can occur within three days and lighten teeth by one or two shades. This type of tooth
whitening is available over-the-counter and professionally from an oral health professional.
Baking soda
Baking soda is a safe, low abrasive, and effective stain removal and tooth whitening toothpaste. Tooth
whitening toothpaste that have excessive abrasivity are harmful to dental tissue, therefore baking soda
is a desirable alternative. To date, clinical studies on baking soda report that there have been no
reported adverse effects. It also contains acid-buffering components that makes baking soda biologically
antibacterial at high concentrations and capable of preventing growth of Streptococcus mutans. Baking
soda might be useful for caries-prone patients as well as those who wish to have whiter teeth.
Now Moving Towards some invasive operative techniques of Esthetic dentistry, treatment modalities which help in changing patients anterior esthetic appearance are:
Dental composite restorations are widely used tooth coloured esthetic restorations and almost
completely replacing old contemporary Silver amalgam and dental cement restorations making Dental
practice Mercury free.
Dental composite resins (better referred to as "resin-based composites" or simply "filled resins") are
types of synthetic resins that are used in dentistry as restorative material or adhesives. Dental
composite resins have certain properties that will benefit patients according to the patient's cavity. It
has a micro-mechanical retention property that makes composite more effective for filling small cavities
where amalgam fillings are not as effective and could therefore fall out (due to the macro-mechanical
retention property of amalgam). Synthetic resins evolved as restorative materials since they were
insoluble, of good tooth-like appearance, insensitive to dehydration, easy to manipulate and reasonably
inexpensive.
Dental composites, or resin-based composites, are synthetic materials that combine polymeric matrix with a dispersion of glass, mineral, or resin filler particles and/or short fibers by coupling agents. Just like dental amalgam, they are used to restore tooth structure lost through trauma, caries, or other diseases. Composites can also be used as cements to cement crowns and veneers, etc. While the amalgam is phasing out in dentistry, composites have become one of the most widely used esthetic restorative materials
As with other composite materials, a dental composite typically consists of a resin-based oligomer
matrix, such as :

Setting mechanisms of resin composite Types of setting mechanisms/Polymerisation:
Resin filler can be made of glasses or ceramics. Glass fillers are usually made of crystalline silica, silicone
dioxide, lithium/barium-aluminium glass, and borosilicate glass containing zinc/strontium/lithium.
Ceramic fillers are made of zirconia-silica, or zirconium oxide.
Fillers can be further subdivided based on their particle size and shapes such as:
Classification of resin composites according to handling characteristics This classification divides resin composite into three broad categories based on their handling characteristics: